Abstract
Introduction: we previously reported the outcome of the DESTINY study, in which patients with CML in chronic phase in at least MMR, were invited to stop treatment only if they remained in at least MMR after a 12 month period of 50% de-escalation of their TKI. Recurrence was defined as confirmed loss of MMR. The 3 year probabilities of recurrence-free survival (RFS) were 72% and 36% in patients who entered the study in MR4 (or deeper) and MMR respectively. We also observed that RFS was better in patients in whom RT-qPCR (IS) ratios did not rise between months 0-12. We now report on one of the secondary outcomes, namely a comparison of standard RT-qPCR and digital droplet (dd) PCR in the prediction of molecular recurrence.
Methods: the study completed recruitment in April 2015 and LPLV was in April 2018. Further follow-up to identify late molecular recurrence occurred in 2021. 157/174 patients discontinued their TKI at month 12, of whom 22 had a RT-qPCR ratio consistent with MMR but not MR4 at month 12. A further 7 patients were lost to follow-up for a variety of reasons. As our hypothesis was that ddPCR might offer enhanced sensitivity over RT-qPCR, we compared samples from patients who stopped their TKI in at least MR4 as defined by RT-qPCR (n=128), and investigated the relationship between the level of response at stopping and subsequent molecular recurrence. ddPCR was used to determine the number of ABL1 and BCR::ABL1 copies for each patient. BCR::ABL1/ABL1 values were calculated from total copy numbers across n=3 technical replicates for each target and results were converted to the International Scale (IS). 2µL cDNA was used per replicate, and primers and probes were per the EAC design previously described (Gabert et al, 2003).
Results: Of 22 patients who had RT-qPCR ratios between 0.01% IS and 0.1% IS (MMR) at month 12, 19 (86%) had molecular recurrence: 5/22 had RT-qPCR ratios consistent with MMR at study entry (month 0) and 2 had recurrence but the remainder experienced increasing RT-qPCR levels over the period of de-escalation and all had molecular recurrence.
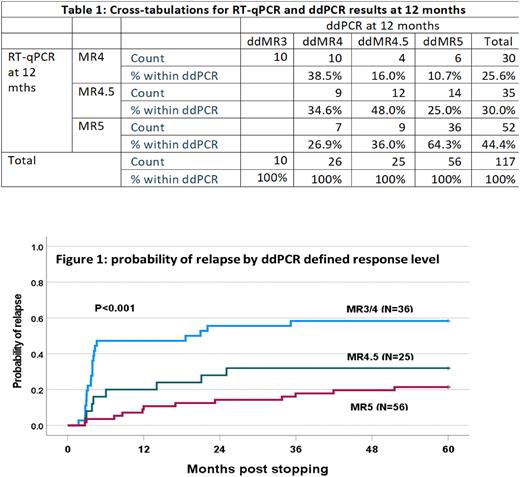
RT-qPCR results were available for all 128 patients in MR4 or better at month 12, but ddPCR results for only 117/128 due to technical failure (n=3) or insufficient residual sample for ddPCR analysis (n=8). There was in general correlation of results between the methodologies but incomplete concordance with ddPCR ratios >RT-qPCR, ddPCR = RT-qPCR and ddPCR < RT-qPCR in 54, 14 and 49 samples respectively. The absolute values for the differences were generally small (median increases of 0.0018 when ddPCR>RT-qPCR and 0.0007 when RT-qPCR>ddPCR) (Table 1). The probabilities of molecular recurrence at 5 years by RT-qPCR defined response levels at study entry of MR4 (n=30), MR4.5 (n=42) and MR5 (n=56) were 50%, 43% and 17% respectively (p<0.001). The equivalent probabilities by ddPCR defined MR3/MR4 (n=36), MR4.5 (n=25) and MR5 (n=56) were 58%, 32% and 21% respectively (p<0.001) (Figure 1). ddPCR reclassified 10 patients in MR4 by RT-qPCR to MMR and 5 of these 10 had recurrence, equivalent to the recurrence rate of ddPCR defined MR4 but considerably lower than RT-qPCR defined MMR.
56 patients were in MR5 by RT-qPCR, of whom 20 were reclassified by ddPCR as MR4.5 (n=11), MR4 (n=6) or MMR (n=3), of whom 5 had recurrence (2 x MR4, 3 x MR4.5). Similarly 56 patients were in MR5 by ddPCR and 20 were reclassified as MR4.5 (n=14) or MR4 (n=6), of whom 6 had recurrence (5 x MR4.5).
Further analyses using receiver operator curves were unable to identify PCR ratios by either method that had sufficient specificity to be used in clinical practice.
Conclusions: Patients in MR5 are at a low risk of disease recurrence. The risk of recurrence was highest in patients in MMR at the time of stopping and for this group, the value of a methodology offering higher sensitivity than standard RT-qPCR is questionable. For patients in deeper response levels, even those in MR5, we could not identify any advantage of ddPCR over RT-qPCR as performed in our laboratories.
Disclosures
Milojkovic:Novartis: Honoraria; Bristol Meyers Squibb: Consultancy, Honoraria; Incyte: Honoraria, Research Funding; Pfizer Inc.: Honoraria. Copland:Cyclacel: Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees; pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria. Cross:Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria; Astellas Pharma: Consultancy, Honoraria. Clark:Pfizer: Honoraria. Apperley:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal